Open call
MedPhab open call programme report
The objective of the Open Call programme was to provide technology development services for companies that are adopting advanced photonics technologies in medical diagnostics solutions. As the technology development services are pioneering in nature, MedPhab provides EU-based financial support for up to 75% of 15 business cases. This programme ran from March 2021 to April 2023, in which all companies that complied with the eligibility criteria were considered for evaluation.
The proposal submission had two stages: a pre-screening phase and a full proposal submission. The pre-screening stage was a continuous process from January 2022 (earlier had defined deadlines), in which applicants could send their in-take form anytime when the calls for demo cases were open. The full proposal stage from January 2022 deadlines were every two months (i.e. end of February 2022, April 2022, June 2022, September 2022, November 2022, February 2023, and April 2023, at 17:00 CET). Before January 2022 only one cut-off during November 2021 took place. All details of how the programme was structured can be found here.
In total, there were 8 cut-offs for full proposals. The budget allocated for the programme was 1.8 M€, and for each case was up to 250 k€ when several MedPhab partners participated as service and technology providers. The maximum support was 125 k€ a single MedPhab partner was involved. Since the launch of the programme, 41 in-take forms from 17 countries were received (see Figure 1 and Figure 2), which translated into 23 full proposals. Finally, 15 projects were initiated. All the applicants were SMEs.
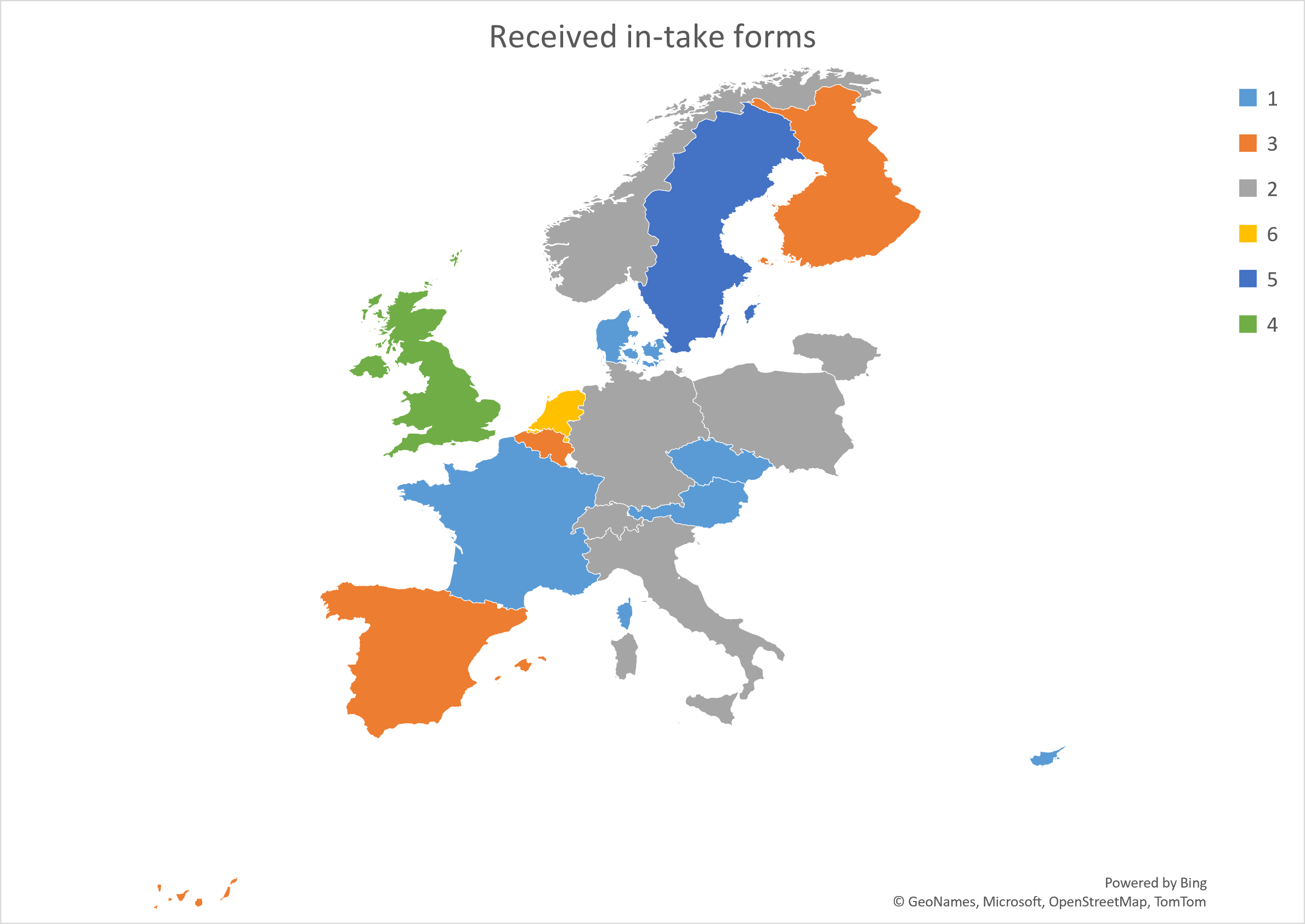
Figure 1. European distribution of the in-take forms received.
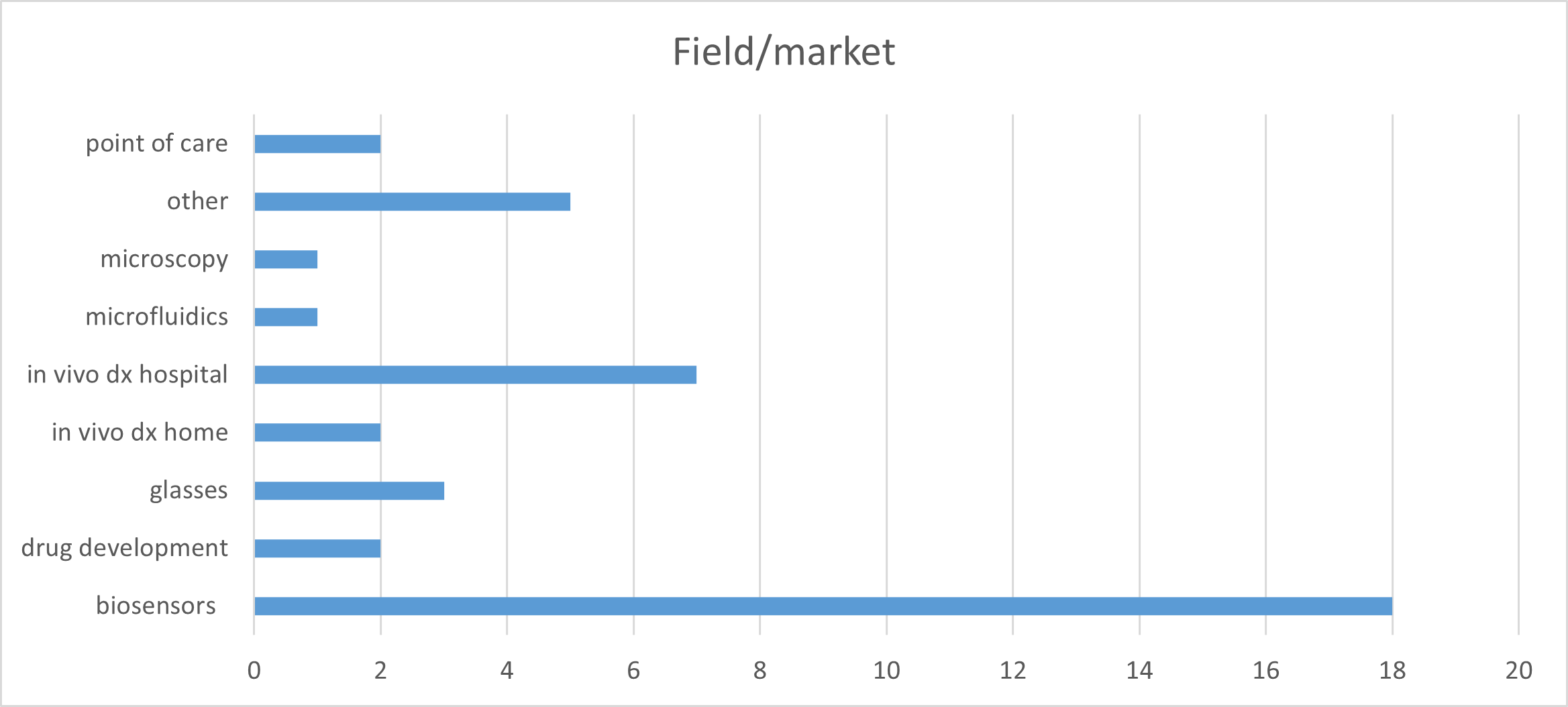
Figure 2. Simplified and categorized field or markets from the companies in-take forms. Dx= diagnostics.
When completing the full proposals, applicants were asked how they learnt about MedPhab. The responses were categorized into four groups: through EPIC (dissemination manager in the project), via the networks, either colleagues recommended, or they worked with a MedPhab partner earlier in another project or initiative, through events (e.g. COMPAMED/MEDICA, MedPhab webinar) and the website. 56% of the companies knew about MedPhab through their networks (Figure 3).
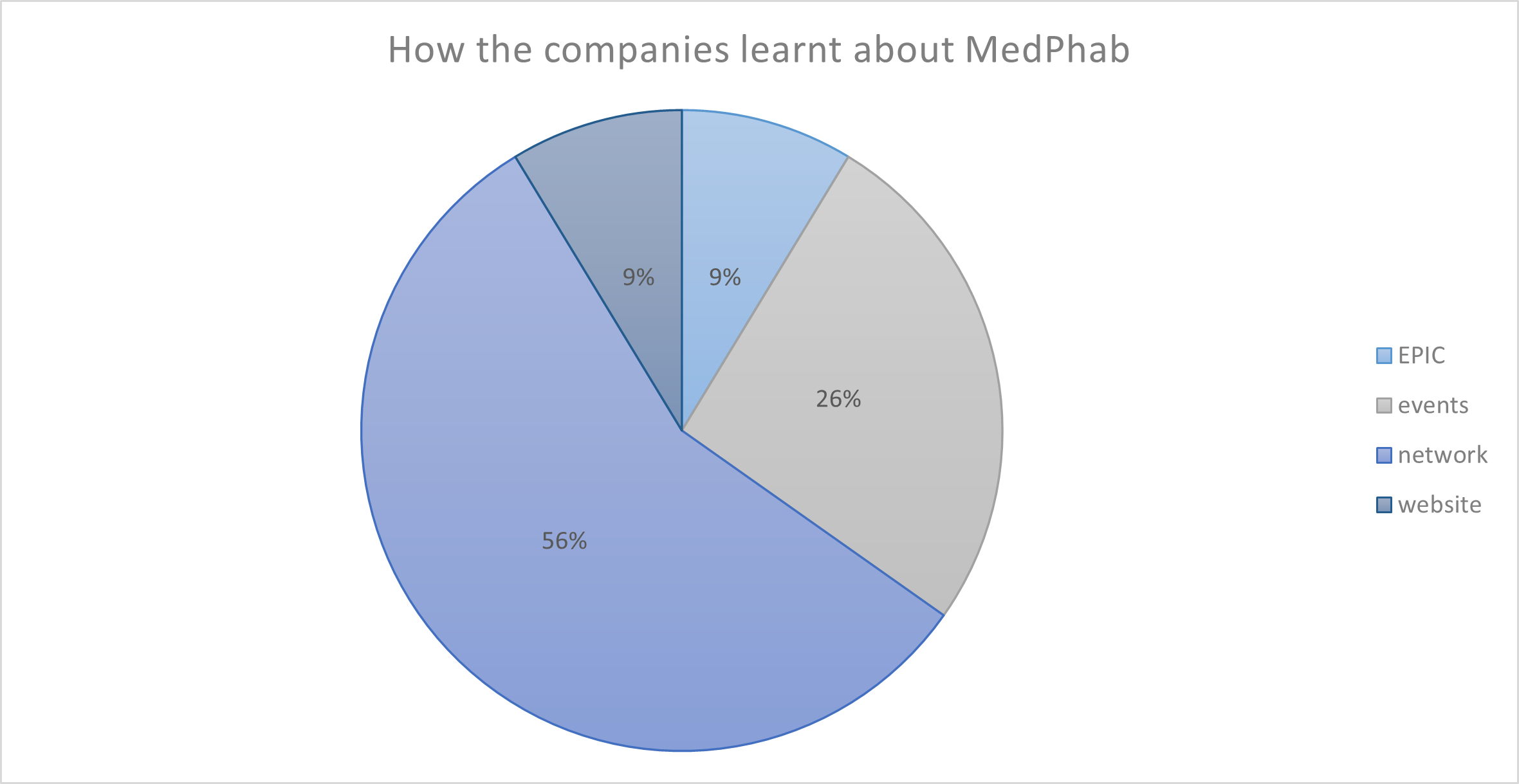
Figure 3. How the companies that applied for full proposals learnt about MedPhab.
This programme provided opportunities to improve the customer service process which is an essential element in the sustainable operation of the MedPhab pilotline. In addition, it has shown what are needs of SMEs in photonics technologies available by MedPhab. Success stories and further information can be found at https://medphab.com/
Summary
Open call for demo cases program (closed)
MedPhab (EU GA871345), Europe’s first pilot line for photonics-based medical devices, is launching an Open Call for the external companies developing medical products. The objective of the Open Call is to provide technology development services for the companies that are adopting advanced photonics technologies in the medical diagnostics solutions. As the technology development services are pioneering in nature, we provide subsidized financial support to up to 20 business cases. For small and medium enterprises (SMEs) the support is 75% of the costs and for large enterprises the support is 50%.
The MedPhab consortium consists of research organizations and industrial parties with ISO13485 certificate allowing the seamless development from early phase proof-of-concept to regulated pilot production. Depending on the phase of the development, the most suitable MedPhab party or parties are selected as developer.
Participation in the open call
The proposal submission has two stages: a pre-screening phase and a full proposal submission (more details in Application Guidelines).
If you are interested in learning more about MedPhab and about how you can participate in the Open Call, you can see the recording here of the Webinar that took place on May 11, 2021. Another webinar was hold on October 28, 2021. More information here. If you would like to know the experience of companies selected in the first cut-offs, register to the Webinar on October 27, 2022 at 16:00 – 17:30 CEST here.
The pre-screening stage is a continuous process from January 2022, in which applicants can send their in-take form anytime in which the calls for demo cases are open.
Full proposal stage: From January 2022 deadlines will be every two months (i.e. end of February 2022, April 2022, June 2022, September 2022, November 2022, February 2023, and April 2023, at 17:00 CET).
Expected duration of participation: 3-12 months
Maximum amount of EU-financial support for each project: 250 K€ (only in multi-party cases), 125K (for single-party cases)*
All the enquiries are handled confidentially. MedPhab Open Call flyer.
Email address for further information and questions: helpdesk@medphab.eu
*Multi-party means several MedPhab partners are involved on the case/project, single-party refers to the participation of a single MedPhab partner on the case/project.
What?
Project acronym: MedPhab Project full name: Photonics Solutions at Pilot Scale for Accelerated Medical Device Development Project grant agreement number: 871345 MedPhab, first European pilot line for photonics-based medical devices, is organizing Open Call for the external companies developing medical products. The objective of the Open Call is to provide technology development services for the companies that are adopting advanced photonics technologies in the medical diagnostics solutions. As the technology development services are pioneering in nature, we provide subsidized financial support to up to 20 business cases. Interested companies can apply for the support to development, manufacturing and upscaling of photonic-based medical devices products. The types of activities that qualify for receiving support must be related to medical device products enabled by one or more following photonics technologies:
-
- Fibre optics
- Microfluidics
- Surface functionalisation
- Instrumentation
- Opto-electronic integration
- Miniaturisation for micromodules and wearables
The aims of Demo Case for these topics will be:
-
-
- Assessment of technology and manufacturing status of medical device concept;
- Execution of experiments to advance the maturity level of your concept;
- Small series fabrication of demonstrators if possible within the scope;
- Elaborate your exploitation plan (e.g. cost calculation, supply chain, market expansion).
-
Typical company profile, one or more can apply:
-
-
- You have a photonics-based innovative medical device concept;
- You are planning to advance your concept towards production readiness;
- You want to set up a manufacturing value chain;
- You need a technology that fits with the technology offerings available in MedPhab.
-
When?
The Call will be open from March 2021 to April 2023, and applicants will be able to apply anytime. The Evaluation process involves two stages: a pre-screening process and a full proposal evaluation.
- The pre-screening stage is a continuous process from January 2022, in which applicants can send their in-take form anytime in which the calls for demo cases are open.
- Full proposal stage: First cut-off deadline: November 30, 2021. From January 2022 deadlines will be every two months (i.e. end of February 2022, April 2022, June 2022, September 2022, November 2022, February 2023, and April 2023, at 17:00 CET).
Who?
The Call is open to:
- Small to Medium Enterprises (SMEs)
- Large companies
Please note that the main factors determining the type of the company are staff headcount and, either, turnover or balance sheet total (see the COMMISSION RECOMMENDATION of 6 May 2003 concerning the definition of micro, small and medium-sized enterprises). If you are not sure, please check the User Guide to the SME Definition. Please also note that a check of the type of company will be assessed if your demo-case is selected for co-funding. 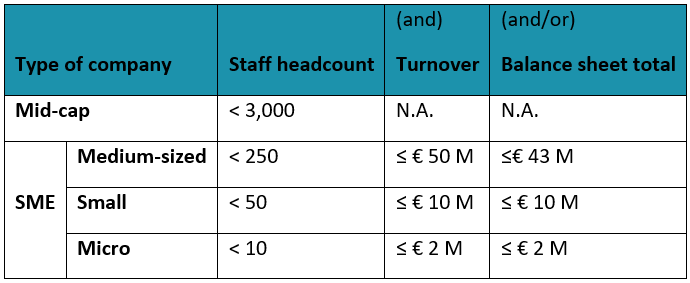
In order to be eligible for this Call, your company will also have to be established in one of the eligible countries.*
*UK is considered as before Brexit.
Why?
As a selected Demo-case company you will:
- Be among the first adopters of open access pilot line for companies that are interested in the manufacture and system-level integration of photonic medical devices towards product launch.
- Have access to MedPhab’s unique ecosystem composed of leading research organizations, several innovative technology suppliers, recognized experts in the business development and investors.
- Have an access to expertise required to manufacture photonic in-vitro and in-vivo medical devices.
- Receive customized support through a unique set of services integrating technical, business, ecosystem expertise which will enable you to build up sustainable business cases.
The Maximum EU-contribution for a Demo case is up to 250 k€ (only in multi-party cases): 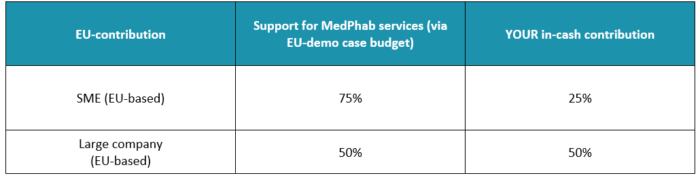
EC-contribution can be up to 250k€ in each project, ONLY when multi-party participation applies (even in first-time projects). This also allows selected companies of previous cut-offs to apply for a second project without exceeding 250k€ of the sum of the two projects. It is required that we have multiple MedPhab parties in the project and 1st project is completed and reported properly prior starting the continuation project.
Process
Please notice that the proposal submission has two stages: a pre-screening phase and a full proposal submission. In both stages the documents should be prepared in English. If the in-take form in the pre-screening phase is not submitted a full proposal cannot be submitted or evaluated. The full proposal will be evaluated by MedPhab evaluators in the nearest cut-off. All proposals will be evaluated (scores ranging from 0 to 5) according to the following sections:
- Concept: This section should cover how this Demo Case aligns to the MedPhab call, the business needs, the technological challenge identified and your approach to innovation.
- Implementation: This section should include details of your company resources, a draft outline of work activities, IPR management and any risks identified.
- Impact: This section describes the key outcomes of your Demo Case and the impact that your innovative Product / series of products will have on the market. How do you propose to grow your business and increase your productivity into the long term as a result of the Demo Case? Describe the importance of MedPhab services. How MedPhab services can accelerate the product development and reduce the costs?
The maximum overall score is 15. The standard threshold for individual criteria is 3, and the standard overall threshold, applying to the sum of the three individual scores, is 10. The average of the 3 scores given by evaluators per section and total will be calculated for each proposal. Proposals failing to achieve the threshold score per individual criteria and the overall threshold will be rejected. Proposals exceeding the threshold points will be ranked using the following weighting ratios between different categories: 40% Concept, 10% Implementation and 50% Impact. About 700k€ of funding has been reserved for the 1st Open call round. The proposals with the highest scores in the ranking will be funded in the descending order. The most successful proposals, having passed the first stage of the pre-screening phase and full proposal evaluation, will be offered the chance to negotiate and define a Joint Implementation Agreement (JIA) with MedPhab Service Delivery Manager and MedPhab partners involved. Evaluation of the full proposal will be returned in 20 natural days from submission deadline. The entire process from in-take form submission (pre-screening) until the end of service delivery could take up to 18 months in the case that your proposal is selected for funding.
Confidentiality At the pre-screening stage no Non-Disclosure Agreements (NDA) will be required. During the proposal writing phase an NDA will be signed between the MedPhab partner(s) involved in writing and the client. Another generic and non-negotiable NDA only for the evaluation purposes will be signed between the MedPhab partners with a representative in the Evaluation Team and the client before the evaluation step. After a successful evaluation of the proposal, an NDA will be signed between the executing partner(s) and the client as part of the Joint Implementation Agreement (JIA).
How?
If you are interested in applying to the Call, please follow the steps listed below before submitting your application.
- Go to medphab.eu.
- Check out the offering and technologies of MedPhab if they fit your needs.
- Download & read thoroughly the open call Application Guidelines.
- Complete MedPhab in-take form for the pre-screening process, HERE (no NDA).
- A MedPhab coach will be assigned to you after the pre-screening stage is successfully passed. (NDA between you and your coach).
- Register for the full proposal via this LINK.
- Download the Full Proposal template.
- Complete the Full proposal template, save it as a PDF file (up to 10 pages), upload it and submit it HERE. (Generic NDA between evaluation team and client).
- Wait for the evaluation process to be completed.
- Sign the Joint Implemenation Agreement with the MedPhab partner(s) involved in the demo-case.
- Start the execution of your demo case.
- Return the feedback form.
Documents
- Application Guidelines.
- MedPhab offering (Handbook)
- In-take form (fill it here), DRAFT (only as reference)
- The full proposal is downloaded and completed through HERE (only after passing the first stage)
- Joint Implementation Agreement
- Non-Disclosure Agreements
Please note that these documents are prepared for the whole procedure, however some might undergo minor changes after the first cut-off. In addition, NDAs (between MedPhab partner and client for preparation of the proposal, and during implementation) and JIA are drafted documents, and will be adapted to each case.
Contact
If you need assistance with applying to the Call, or any other clarification related with MedPhab call, please send us your enquiries by phone to: +420 226 217 422 or by email to helpdesk@medphab.eu . Helpdesk will be active Monday – Friday from 9:00 to 17.00 (CET). We will be happy to help you. We encourage all applicants to revise the technical and business feasibility of their ideas well in advance of submission.
Public information and Feedback

**excluding those specifically mentioned by the client in the in-take form
NOTE: If no concerns are raised by Applicants/clients, they will be asked to fill a Feedback form/Questionnaire in which they will describe the key achievements/results of the demo-case, experience of working with MedPhab, how MedPhab helped in solving the problem, etc. This document will be shared only amongst the MedPhab partners and European Commission.
Complaints
Upon reception of the feedback of the proposal, applicants may submit complaints related to the proposal evaluation and selection to the MedPhab Evaluation Team. The complaints shall address the procedural shortcomings and – in rare cases – factual errors and they are not meant to call into question the decision made by the evaluators.
The complaints should be submitted within 7 working days from the date of the evaluation feedback sent by the MedPhab Helpdesk. If a complaint is submitted after the deadline it will be rejected without further examination.
Complaints sent by applicants must be:
- related to the evaluation process;
- sent by email to MedPhab Helpdesk (helpdesk@medphab.eu);
- received within 7 working days after evaluation feedback sent.
An reply will be sent to complainants no later than two weeks after the submission of the complaints.
A to examine the evaluation process for the case in question. The committee’s role is to ensure a coherent interpretation of complaints and an equal treatment of applicants. The complaint committee, however, will not re-evaluate the proposal, but it will examine the admissibility of the complaints, the legality of the actions against which the complaints are launched and factual arguments and claims of the complaints. Depending on the nature of the complaint, the committee may review the evaluation report and individual comments. In the light of its review, the committee will recommend a course of action. If there is clear evidence of a shortcoming that could affect the funding decision, it is possible that all or part of the proposal will be re-evaluated.
Please note:
- This procedure is concerned with the evaluation process;
- The committee will not call into question the judgment of the individual evaluators;
- A re-evaluation will only be carried out if there is evidence of a shortcoming that affected the quality assessment of a proposal.
- The evaluation score following any re-evaluation will be regarded as definitive. It may be lower than the original score;
- Only one complaint submission per application will be considered by the committee;
- All complaints will be treated confidentially.
